2. Acids, Bases and Salt
Aquaregia:
We know that metals like gold and silver are not reactive with either HCl or HNO3. But the mixture of these two acids can dissolve gold. This mixture is called Aquaregia. It is a mixture of hydrochloric acid and nitric acid prepared optimally in a molar ratio of 3:1. It is a yellow-orange fuming liquid. It is a highly corrosive liquid, able to attack gold and other substances.
Chemical formula: 3 HCl + HNO3
Solubility in Water: Miscible in water
Melting point: – 42°C (- 44°F, 231K)
Boiling point: 108°C (226°F, 381K)
The term aquaregia is a Latin phrase meaning ‘King’s Water’. The name reflects the ability of aquaregia to dissolve the noble metals such as gold, platinum and palladium.
Uses of Aquaregia:
- It is used chiefly to dissolve metals such as gold and platinum.
- It is used for cleaning and refining gold.
Neutralisation Reaction:
When neutrality is achieved between two different chemical substances with different chemical properties through a reaction then it is called neutralization in chemistry. Thus neutralization is a chemical reaction in which an acid and a base react with each other to form salt and water. Neutralization reaction between an acid and a base can be written as:
Acid + Base → Salt + Water
In this reaction, H+ and Cl– ions are produced by the hydrochloric acid and Na+ and OH– ions are produced by sodium hydroxide (base). When these ions combine together sodium chloride (NaCl) salt and water are produced.
Similarly other acids also produce their salts when they react with bases. Some of the salts produced by neutralization reaction.
Acid | Base | Salt |
Hydrochloric acid HCl | Sodium hydroxide NaOH | Sodium chloride NaCl |
Sulphuric acid H2 SO4 | Sodium hydroxide NaOH | Sodium sulphate Na2 SO4 |
Nitric acid HNO3 | Sodium hydroxide NaOH | Sodium nitrate NaNO3 |
Acetic acid CH3 COOH | Sodium hydroxide NaOH | Sodium acetate CH3 COONa |
Neutralisation reactions in our daily life:
Balancing acids and bases is important for our health and for our environment. We come across various neutralization reactions in our daily life. Let us study about the importance of some of those reactions.
Bee bite:
Whenever bees or red ants bite us they inject an acid called formic acid into our body. This acid cause burning sensation and pain. To suppress the pain a suitable base in the form of calcium hydroxide (lime paste available at home) is applied so as to neutralise the formic acid.
Wasp bite:
When we are bitten by wasp, we feel the burning sensation and pain. It is due to an alkaline substance injected by the insect. To neutralise the alkalinity we use vinegar which is an acid.
Tooth decay:
Generally it is advised by the doctors that we should brush our teeth twice a day. This is because the bacteria present in our mouth decompose the food particles stuck in the gaps between our teeth thereby causing acid formation which leads to tooth decay. To prevent this we have to neutralize the acid. When we brush with tooth powder or tooth paste containing weak bases, the acid gets neutralized. So our teeth will be strong and healthy.
Acidity:
As we know, hydrochloric acid present in our stomach helps the digestion of food material along with the enzymes secreted by liver, gallbladder and pancreas. Sometimes due to excessive production of hydrochloric acid in our stomach we feel burning sensation in food pipe and in chest area. If this happens again and again ulcer will be formed in stomach and food pipe, which further aggravates the conditions. In order to neutralize, antacids which are nothing but weak bases like aluminum and magnesium hydroxides are used. As a result the acidity is removed.
Agriculture:
Acidic soil is not suitable for plant growth. So farmers add lime fertilisers such as powdered lime (CaO), limestone (CaCO3) or ashes of burnt wood to the soil to neutralise the acidity.
Industries Effluents from the industries contain acids such as sulphuric acid. It is treated by adding lime to neutralise it before it is discharged into rivers and streams. Similarly, in power stations fossil fuels such as coal are burnt to produce electricity. Burning fossil fuels will liberate sulphur dioxide gas as an acidic pollutant in the air. Hence, power stations treat this acidic gas using powdered lime (CaO) or limestone (CaCO3) to neutralise it so that air pollutant can be prevented.
Indicators:
An indicator or acid– base indicator is a chemical substance which indicates the acidic or basic nature of a solution by suitable colour change. These may be natural or synthetic.
Natural indicators:
Natural indicators are chemical substances which are obtained from the natural resources. Litmus, turmeric juice, China rose petals, red cabbage, grape juice and beetroot juice are the indicators obtained from natural resources.
Turmeric indicator:
By adding small amount of water to turmeric powder a paste is prepared. This is applied on a blotting paper or filter paper and dried. These strips are used as indicators to find the nature of the solution. In acidic solution turmeric indicator paper has no change in colour. That means it remains yellow. In basic solution the colour changes from yellow to red.
Hibiscus flower indicator:
Some hibiscus flowers soaked in warm water for about 5 to 10 minutes forms a solution. This solution can be used as indicator. In acidic solution, the colour will be changed to deep pink or deep red. In basic solution, the colour will be changed into green.
Beet root juice indicator:
Extracts of beet root are also used as an indicator for identifying the acidic or basic nature of a solution.
Litmus:
Litmus is the most common indicators used in the laboratories. Litmus is a natural indicator which is extracted from lichens.
It is available in the form of solution or in the form of strips prepared by absorbing litmus solution on filter paper. It is either red or blue in colour. Blue litmus paper turns red in acidic solution and red litmus paper turns blue in the basic solution.
Synthetic indicators:
An indicator prepared from artificial substances is known as synthetic indicators. Phenolphthalein and methyl orange are the examples for synthetic indicators.
Phenolphthalein:
Phenolphthalein is a colourless compound. Its alcoholic form is used as an indicator. It is colourless in acidic solution but turns pink in basic solution.
Methyl orange:
Solid methyl orange is dissolved in hot water and its filtrate is used as an indicator. It turns red in acidic solution and yellow in basic solution.
The following table gives the colour changes of different indicators in acidic and basic medium.
Indicator | Acidic solution | Basic solution |
Blue litmus | Red | No change in colour |
Red litmus | No change in colour | Blue |
Phenolphthalein | Colourless | Pink |
Methyl orange | Red | Yellow |
Ionic Product of Water:
Although pure water is often considered as a non-conductor of electricity, precise measurements show that it conducts electricity to a little extent. This conductivity of water has resulted from the self-ionisation of water. Self-ionisation or auto ionisation is a reaction in which two like molecules react to give ions. In the process of ionisation of water, a proton from one water molecule is transferred to another water molecule leaving behind an OH— ion. The proton gets dissolved in water forming the hydronium ion as shown in the following equation:
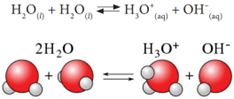
The hydronium ion formed is a strong acid and the hydroxyl ion is a strong base. So as fast as they are formed, they react again to produce water. Thus, it is a reversible reaction and attains equilibrium very quickly. So, the extent of ionisation is very little and the concentration of the ions produced is also very less. The product of the concentration of the hydronium ion and the hydroxyl ion is called ‘ionic product of water’. It is denoted as ‘Kw’. It is mathematically expressed as follows:
![]()
[H3O+] may be simply written as [H+]. Thus the ionic product of water may also be expressed as
![]()
Its unit is mol2 dm-6. At 25° C, its value is 1.00 × 10—14.
Role of pH in Everyday Life:
Our body works within the pH range of 7.0 to 7.8. Living organisms can survive only in a narrow range of pH change. Different body fluids have different pH values. For example, pH of blood is ranging from 7.35 to 7.45. Any increase or decrease in this value leads to diseases. The ideal pH for blood is 7.4.
pH in our digestive system:
It is very interesting to note that our stomach produces hydrochloric acid. It helps in the digestion of food without harming the stomach. During indigestion the stomach produces too much acid and this causes pain and irritation. pH of the stomach fluid is approximately 2.0.
pH changes as the cause of tooth decay:
pH of the saliva normally ranges between 6.5 to 7.5. White enamel coating of our teeth is calcium phosphate, the hardest substance in our body. When the pH of the mouth saliva falls below 5.5, the enamel gets weathered. Toothpastes, which are generally basic are used for cleaning the teeth that can neutralise the excess acid and prevent tooth decay.
pH of soil:
In agriculture, the pH of the soil is very important. Citrus fruits require slightly alkaline soil, while rice requires acidic soil and sugarcane requires neutral soil.
pH of rain water:
The pH of rain water is approximately 7, which means that it is neutral and also represents its high purity. If the atmospheric air is polluted with oxide gases of sulphur and nitrogen, they get dissolved in the rain water and make its pH less than 7. Thus, if the pH of rain water is less than 7, then it is called acid rain. When acid rain flows into the rivers it lowers the pH of the river water also.
The survival of aquatic life in such rivers becomes difficult.
Principle of Acids and Bases:
Arrhenius Concept:
One of the earliest theories about acids and bases was proposed by Swedish chemist Svante Arrhenius. According to him, an acid is a substance that dissociates to give hydrogen ions in water. For example, HCl, H2SO4 etc., are acids. Their dissociation in aqueous solution is expressed as
![]()
The H+ ion in aqueous solution is highly hydrated and usually represented as H3O+, the simplest hydrate of proton [H(H2O)] + . We use both H+ and H3O+ to mean the same.
Similarly a base is a substance that dissociates to give hydroxyl ions in water. For example, substances like NaOH, Ca (OH) 2 etc., are bases.
![]()
Limitations of Arrhenius concept:
- Arrhenius theory does not explain the behaviour of acids and bases in non aqueous solvents such as acetone, Tetrahydrofuran etc…
- This theory does not account for the basicity of the substances like ammonia (NH3) which do not possess hydroxyl group.
Lowry – Bronsted Theory (Proton Theory):
In 1923, Lowry and Bronsted suggested a more general definition of acids and bases. According to their concept, an acid is defined as a substance that has a tendency to donate a proton to another substance and base is a substance that has a tendency to accept a proton from other substance. In other words, an acid is a proton donor and a base is a proton acceptor.
When hydrogen chloride is dissolved in water, it donates a proton to the later. Thus, HCl behaves as an acid and H2O is base. The proton transfer from the acid to base can be represented as
![]()
When ammonia is dissolved in water, it accepts a proton from water. In this case, ammonia (NH3) acts as a base and H2O is acid. The reaction is represented as
![]()
Let us consider the reverse reaction in the following equilibrium
![]()
H3O+ donates a proton to Cl– to form HCl i.e., the products also behave as acid and base.
In general, Lowry – Bronsted (acid – base) reaction is represented as
![]()
The species that remains after the donation of a proton is a base (Base1) and is called the conjugate base of the Bronsted acid (Acid1). In other words, chemical species that differ only by a proton are called conjugate acid – base pairs.
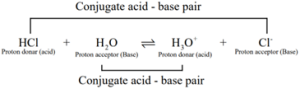
HCl and Cl– , H2O and H3O+ are two conjugate acid – base pairs. i.e., Cl- is the conjugate base of the acid HCl. (or) HCl is conjugate acid of Cl– . Similarly H3O+ is the conjugate acid of H2O.
Limitations of Lowry – Bronsted theory:
- Substances like BF3, AlCl3, that do not donate protons are known to behave as acids.
Lewis Concept:
In 1923, Gilbert. N. Lewis proposed a more generalised concept of acids and bases. He considered the electron pair to define a species as an acid (or) a base. According to him, an acid is a species that accepts an electron pair while base is a species that donates an electron pair. We call such species as Lewis acids and bases.
A Lewis acid is a positive ion (or) an electron deficient molecule and a Lewis base is an anion (or) neutral molecule with at least one lone pair of electrons.
Les we consider the reaction between Boron tri fluoride and ammonia
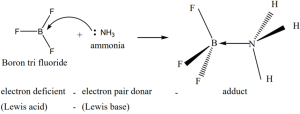
Here, boron has a vacant 2p orbital to accept the lone pair of electrons donated by ammonia to form a new coordinate covalent bond. We have already learnt that in coordination compounds, the Ligands act as a Lewis base and the central metal atom or ion that accepts a pair of electrons from the ligand behaves as a Lewis acid.
Lewis acids | Lewis bases |
Electron deficient molecules such as BF3, AlCl3, BeF2 etc… | Molecules with one (or) more lone pairs of electrons. NH3 , H2O,R-O-H, R-O-R, R – NH2 |
All metal ions Examples: Fe2+, Fe3+, Cr3+, Cu2+ etc… | All anions F– , Cl–, CN–, SCN–, SO42– etc… |
Molecules that contain a polar double bond Examples: SO2, CO2, SO3 etc… | Molecules that contain carbon – carbon multiple bond Examples: CH2 =CH2, CH ≡ CH etc… |
Molecules in which the central atom can expand its octet due to the availability of empty d – orbitals Example: SiF4, SF4, FeCl3 etc… | All metal oxides CaO, MgO, Na2O etc… |
Carbonium ion (CH3)3 C+ | Carbanion CH3− |
Common Ion Effect:
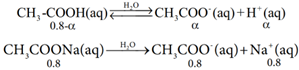
When a salt of a weak acid is added to the acid itself, the dissociation of the weak acid is suppressed further. For example, the addition of sodium acetate to acetic acid solution leads to the suppression in the dissociation of acetic acid which is already weakly dissociated. In this case, CH3COOH and CH3COONa have the common ion, CH3COO–
Let us analyse why this happens. Acetic acid is a weak acid. It is not completely dissociated in aqueous solution and hence the following equilibrium exists.
![]()
However, the added salt, sodium acetate, completely dissociates to produce Na+ and CH3COO– ion.
![]()
Hence, the overall concentration of CH3COO– is increased, and the acid dissociation equilibrium is disturbed. We know from Le chatelier’s principle that when a stress is applied to a system at equilibrium, the system adjusts itself to nullify the effect produced by that stress. So, inorder to maintain the equilibrium, the excess CH3COO– ions combines with H+ ions to produce much more unionized CH3COOH i.e, the equilibrium will shift towards the left. In other words, the dissociation of CH3COOH is suppressed. Thus, the dissociation of a weak acid (CH3COOH) is suppressed in the presence of a salt (CH3COONa) containing an ion common to the weak electrolyte. It is called the common ion effect.
Buffer Solution:
Do you know that our blood maintains a constant pH, irrespective of a number of cellular acid – base reactions? Is it possible to maintain a constant hydronium ion concentration in such reactions? Yes, it is possible due to buffer action.
Buffer is a solution which consists of a mixture of a weak acid and its conjugate base (or) a weak base and its conjugate acid. This buffer solution resists drastic changes in its pH upon addition of a small quantities of acids (or) bases, and this ability is called buffer action. The buffer containing carbonic acid (H2CO3) and its conjugate base HCO3 – is present in our blood. There are two types of buffer solutions.
- Acidic buffer solution: a solution containing a weak acid and its salt.
Example: solution containing acetic acid and sodium acetate
- Basic buffer solution: a solution containing a weak base and its salt.
Example: Solution containing NH4OH and NH4Cl
Buffer action:
To resist changes in its pH on the addition of an acid (or) a base, the buffer solution should contain both acidic as well as basic components so as to neutralize the effect of added acid (or) base and at the same time, these components should not consume each other.
Let us explain the buffer action in a solution containing CH3COOH and CH3COONa. The dissociation of the buffer components occurs as below.
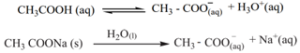
If an acid is added to this mixture, it will be consumed by the conjugate base CH3COO– to form the undissociated weak acid i.e, the increase in the concentration of H+ does not reduce the pH significantly.
![]()
If a base is added, it will be neutralized by H3O+, and the acetic acid is dissociated to maintain the equlibrium. Hence the pH is not significantly altered.
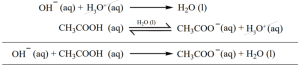
These neutralization reactions are identical to those reactions that we have already discussed in common ion effect.
Les us analyse the effect of the addition of 0.01 mol of solid sodium hydroxide to one litre of a buffer solution containing 0.8 M CH3COOH and 0.8 M CH3COONa. Assume that the volume change due to the addition of NaOH is negligible. (Given: Ka for CH3COOH is 1.8 × 10-5)
The dissociation constant for CH3COOH is given by
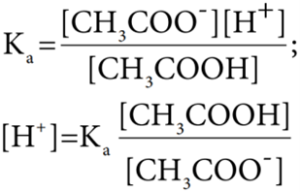
The above expression shows that the concentration of H+ is directly proportional to
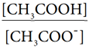
Let the degree of dissociation of CH3COOH be α then,
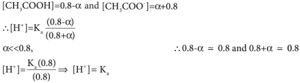
Given that
K for CH COOH is 1.8 10-5
∴ [H+] = 1.8 x 10-5; pH = – log (1.8 x 10-5)
= 5 – log 1.8
= 5 – 0.26
pH = 4.74
Calculation of pH after adding 0.01 mol NaOH to 1 litre of buffer.
Given that the volume change due to the addition of NaOH is negligible ∴ [OH–] = 0.01M. The consumption of OH– are expressed by the following equations.
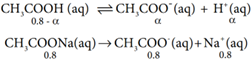
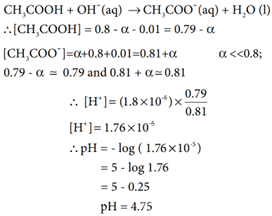
The addition of a strong base (0.01 M NaOH) increased the pH only slightly ie. From 4.74 to 4.75. So, the buffer action is verified.
Calculation:
The pH is the negative logarithm of the hydrogen ion concentration
pH = –log10 [H+]
Example: Calculate the pH of 0.01 M HNO3?
Solution:
[H+] = 0.01
pH = –log10 [H+]
pH = –log10 [0.01]
pH = –log10 [1 × 10-2 ]
pH = –(log101 – 2 log1010)
pH = 0 + 2 × log1010
pH = 0 + 2 × 1 = 2 pH = 2
pOH: The pOH of an aqueous solution is realted to the pH.
The pOH is the negative logarithm of the hydroxyl ion concentration
pOH = –log10[OH–]
Example: The hydroxyl ion concentration of a solution is 1 × 10-9 M. What is the pOH of the solution?
Solution:
pOH = –log10 [OH– ]
pOH = –log10 [1 × 10-9]
pOH = –(log10 1.0 + log10 10-9 )
pOH = –(0–9 log1010)
pOH = –(0 – 9)
pOH = 9
Relationship between pH and pOH:
The pH and pOH of a water solution at 25oC are related by the following equation.
pH + pOH = 14
If either the pH or the pOH of a solution is known, the other value can be calculated.
Example: A solution has a pOH of 11.76. What is the pH of this solution?
pH = 14 – pOH
pH = 14 – 11.76 = 2.24
Problems:
Example 1: Calculate the pH of 0.001 molar solution of HCl.
Solution: HCl is a strong acid and is completely dissociated in its solutions according to the process:
From this process it is clear that one mole of HCl would give one mole of H+ ions. Therefore, the concentration of H+ ions would be equal to that of HCl, i.e., 0.001 molar or 1.0 × 10–3 mol litre–1.
Thus, [H+] = 1 × 10–3 mol litre–1
pH = –log10[H+] = –log1010–3
= –(–3 × log10) = –(3 × 1) = 3
Thus, pH = 3
Example 2: What would be the pH of an aqueous solution of sulphuric acid which is 5 × 10–5 mol litre–1 in concentration.
Solution: Sulphuric acid dissociates in water as:
Each mole of sulphuric acid gives two mole of H+ ions in the solution. One litre of H2 SO4 solution contains 5 × 10–5 moles of H2SO4 which would give 2 × 5 × 10–5 = 10 × 10–5 or 1.0 × 10–4 moles of H+ ion in one litre of the solution.
Therefore,
[H+] = 1.0 × 10–4 mol litre–1
pH = –log10[H+] = –log1010–4 = –(–4 × log1010)
= –(–4 × 1) = 4
Example 3: Calculate the pH of 1 × 10–4 molar solution of NaOH.
Solution: NaOH is a strong base and dissociates in its solution as:
One mole of NaOH would give one mole of OH– ions. Therefore,
[OH–] = 1 × 10–4 mol litre–1
pOH = –log10[OH–] = –log10 × [10–4]
= –(–4 × log1010)= –(–4) = 4
Since, pH + pOH = 14
pH = 14 – pOH = 14 – 4 = 10
Example 4: Calculate the pH of a solution in which the concentration of the hydrogen ions is 1.0 × 10–8 mol litre–1.
Solution: Here, although the solution is extremely dilute, the concentration given is not of an acid or a base but that of H+ ions. Hence, the pH can be calculated from the relation:
pH = –log10[H+]
given [H+] = 1.0 × 10–8 mol litre–1
pH = –log1010–8 = –(–8 × log1010)
= –(–8 × 1) = 8
Example 5: If the pH of a solution is 4.5, what is its pOH?
Solution:
pH + pOH = 14
pOH = 14 – 4.5 = 9.5
pOH = 9.5
