1. Elements and Compounds
Elements:
Elements are everywhere. They are the building blocks of everything on Earth: pencil, desk, mountains, car, book, etc. Do you know that when you are breathing you are actually inhaling air? The air you breathe is made up of many elements like oxygen, nitrogen and argon.
An element is a pure substance that cannot be broken down by chemical methods into simpler components. For example, the element gold cannot be broken down into anything other than gold. If you keep hitting gold with a hammer, the pieces would get smaller, but each piece will always be gold.
Elements consist of only one type of atoms. An atom is the smallest particle of an element that still has the same properties of that element.
All atoms of a specific element have exactly the same chemical makeup, size, and mass. Each atom has an atomic number, which represents the number of protons that are in the nucleus of a single atom of that element. There are a total of 118 elements. Many elements occur naturally on Earth; however, some are created in laboratory by scientists.
Metals:
Iron, copper, gold, silver, etc. that we use in our daily life are metals. The properties and uses of metals are given below.
Physical properties of Metals:
Metals are solid under normal conditions of temperature and pressure.
Most metals are hard.
All metals are shiny. The typical shine of metals is called metallic lustre.
Metals generally have high density.
Metals in general have high melting point and boiling point.
Metals can be hammered into very thin sheets. This tendency of metals is called malleability. Using this property aluminum is transformed into silvery foils.
Metals can be drawn into thin wires. This property of metals is called ductility. Example: Copper wires.
Generally metals are good conductors of heat and electricity.
On being hit, metals produce a typical sound. Hence, they are said to be sonorous. This property is being made used in making temple bells.
Uses of Metals:
Iron is used for making bridges, engine parts, iron-sheets and bars.
Copper is used for making electrical wires, coins and statue.
Silver and gold are used for making jewels, and for decorative purposes and photography.
Mercury is used in thermometers and barometers because of its high density and uniform expansion at different temperature.
Aluminium is used in electrical wires, cables and in aerospace industries.
Lead is used in automobile batteries, X-ray machines.
Non-Metals:
Elements like sulphur, carbon, oxygen etc. are non-metals. Some of the properties and uses of non-metals are given below.
Properties of Non-metals:
Non-metals occur as solids, liquids or gases at normal temperature. For example, sulphur and phosphorus occur in solid state while bromine occurs in liquid state. Elements like oxygen, nitrogen etc., occur in gaseous state.
Non-metals are generally not hard except diamond ( a form of carbon).
Non-metals have a dull appearance.
Non-metals are generally soft and have low densities. The exception here is diamond (a form of carbon) which is the hardest naturally occurring substance.
Non-metals have low melting point and boiling point.
Non-metals are non-malleable. ¾ Non-metals are not ductile. Carbon fibre is highly ductile.
Non-metals are generally bad conductors of electricity. Graphite (a form of carbon) is an exception.
Non-metals do not produce sound (non-sonorous) when hit.
Uses of Non-metals:
Diamond (a form of carbon) is used for making jewels, cutting and grinding equipments. Graphite is used in making pencil lead.
Sulphur is used in the manufacturing of gun powder and vulcanization of rubber.
Phosphorus is used to make match boxes, rat poison etc.
Nitrogen is used for manufacturing ammonia.
Chlorine is used as a bleaching agent and in sterilizing water.
Hydrogen is used as a rocket fuel and hydrogen flame is used for cutting and welding purposes. Hydrogen is also used as a reducing agent.
Difference between Metals and Non-metals:
Property | Metal | Metal |
Physical state at room temperature | Usually solid (Occasionaly liquid) | Solid, liquid or gas |
Malleablity | Good | Poor (Usually soft or brittle) |
Ductility | Good | Poor (Usually soft or brittle) |
Melting point | Usually high | Usually low |
Boiling point | Usually high | Usually low |
Density | Usually high | Usually low |
Conductivity (Thermal and Electrical) | Good | Very poor |
Metalloids:
The elements which exhibit the properties of metals as well as non-metals are called metalloids. Examples: Boron, Silicon, Arsenic, Germanium, Antimony, Tellurium and Polonium.
Physical properties of Metalloids:
Metalloids are solids at room temperature.
They can form alloys with other metals.
Some metalloids, such as silicon and germanium, can act as electrical conductors under specific conditions. Thus, they are called semiconductors.
Silicon which is a metalloid appears lustrous, but it is neither malleable nor ductile. It is brittle – a characteristic of some non metals. It is a much poorer conductor of heat and electricity than the metals.
The physical properties of metalloids tend to be metallic, but their chemical properties tend to be non-metallic.
Uses of Metalloids:
Silicon is used in electronic devices.
Boron is used in fireworks and as a fuel for ignition in rocket.
Name of elements with atomic number above 100:
The name was derived directly from the atomic number of the new element using the following numerical roots.
Digit | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
Root | nil | un | bi | tri | quad | pent | hex | sept | oct | enn |
Abbreviation | n | u | b | t | q | p | h | s | o | e |
The numerical roots corresponding to the atomic number are put together and ‘ium’ is added as suffix
The final ‘n’ of ‘enn’ is omitted when it is written before ‘nil’ (enn + nil = enil) similarly the final ‘i’ of ‘bi’ and ‘tri’ is omitted when it written before ‘ium’ (bi + ium = bium; tri + ium = trium)
The symbol of the new element is derived from the first letter of the numerical roots.
Atomic number | Temporary Name | Temporary Symbol | Name of the element | Symbol |
101 | Unnilunium | Unu | Mendelevium | Md |
102 | Unnilbium | Unb | Nobelium | No |
103 | Unniltrium | Unt | Lawrencium | Lr |
104 | Unnilquadium | Unq | Rutherfordiium | Rf |
105 | Unnilpentium | Unp | Dubnium | Db |
106 | Unnilhexium | Unh | Seaborgium | Sg |
107 | Unnilseptium | Uns | Bohrium | Bh |
108 | Unniloctium | Uno | Hassium | Hs |
109 | Unnilennium | Une | Meitnerium | Mt |
110 | Ununnilium | Uun | Darmstadtium | Ds |
111 | Unununium | Uuu | Roentgenium | Rg |
112 | Ununbium | Uub | Copernicium | Cn |
113 | Ununtrium | Uut | Nihonium | Nh |
114 | Ununquadium | Uuq | Flerovium | Fl |
115 | Ununpentium | Uup | Moscovium | Mc |
116 | Ununhexium | Uuh | Livermorium | Lv |
117 | Ununseptium | Uus | Tennessine | Ts |
118 | Ununoctium | Uuo | Oganesson | Og |
Periodic Trends in Properties:
The electronic configurations of elements help us to explain the periodic recurrence of physical and chemical properties. Anything which repeats itself after a regular interval is called periodic and this behaviour is called periodicity. Some of the atomic properties of the elements are periodic.
Properties such as atomic radius, ionic radius, ionisation energy, electronegativity, electron affinity, show a regular periodicity and hence they are called periodic properties. The main significance of the modern periodic table is that it gives a clear understanding of the general properties and trends within a group or a period to predict with considerable accuracy, the properties of any element, even though that element may be unfamiliar to us. Let us discuss the periodic trend of some of the properties.
Atomic Radius:
Atomic radius of an atom is defined as the distance between the centre of its nucleus and the outermost shell containing the valence electron.
Example: The experimental internuclear distance in Cl2 molecule is 1.98 Å. The covalent radius of chlorine is calculated as below:
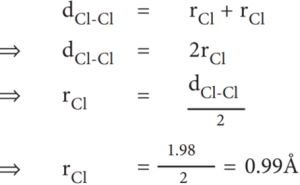
Atomic radius of an atom is defined as the distance between the centre of its nucleus and the outermost shell containing the valence electron. Direct measurement of the radius of an isolated atom is not possible. Except for noble gases, usually the atomic radius is referred to as covalent radius or metallic radius depending on the nature of the bonding between the concerned atoms. Atomic radius in metal atoms is known as metallic radius. It is defined as half the distance between the nuclei of adjacent metal atoms. In non-metallic elements, their atomic radius is known as covalent radius. It is defined as half the distance between the adjacent nuclei of two covalently bonded atoms of the same element in a molecule. For example, let us consider H2 molecule. The distance between the two hydrogen nuclei of the molecule is 0.74 Å. So its covalent radius is 0.74/2 = 0.37 Å.
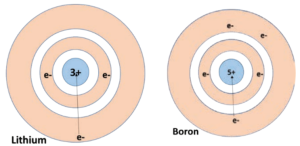
When you look at the variation of the atomic radii in the periodic table, there are two distinct trends. Along the period, from left to right, the atomic radius of the elements decreases whereas along the groups, from the top to bottom, the atomic radius increases. The increase, down a group, is due to the increase in the valence shell number down the group. As the shell number increases, the distance between the valence shell and the nucleus increases. In contrast, when you observe along the period, the shell number remains the same but the number of protons (i.e. atomic number) increases. More and more positive charges impose a strong attraction over the electrons and thus the electron cloud shrinks towards the nucleus, which results in the decrease in the atomic size. Figure 8.4 shows how the atomic radius decreases from lithium to boron.
Ionic Radius:
It is defined as the distance from the centre of the nucleus of the ion upto the point where it exerts its influence on the electron cloud of the ion.
Pauling Method:
Pauling assumed that ions present in a crystal lattice are perfect spheres, and they are in contact with each other. Therefore,
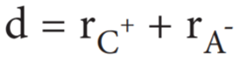
Where d is the distance between the centre of the nucleus of cation C+ and anion A– and rC+, rA– are the radius of the cation and anion respectively.
Pauling also assumed that the radius of the ion having noble gas electronic configuration (Na+ and Cl– having 1s2 2s2 , 2p6 configuration) is inversely proportional to the effective nuclear charge felt at the periphery of the ion.
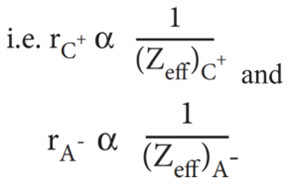
Where Zeff is the effective nuclear charge and Zeff = Z – S
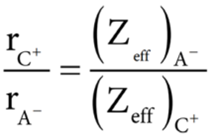
On solving equation values of rC+ and rA– can be obtained
For instance, lithium and sodium lose the single electron from their outermost energy level to form cations. The ions so formed are smaller because the remaining electrons are at a inner cells and attracted more strongly by the nucleus. Fluorine and chlorine become negative ions by gaining an electron. When electrons are added, the charge on the nucleus is not great enough to hold the increased number of electrons as closely as it holds the electrons in the neutral atom. So, as seen in atomic radius, ionic radii also decrease along the period from left to right and increase down the group.
You know that ions are formed when an atom lose or gain electrons. When a neutral atom loses an electron, it becomes a positively charged ion called cation, whereas the gain of an electron by a neutral atom forms a negatively charged ion called anion. The size of the ions is important to determine their behaviours in solutions and the structure of ionic solids. The size of a cation is always smaller than its corresponding neutral atom. But, the anion is larger than its neutral atom.
Note:
As the positive charge increases the size of the cation decreases
As the negative charge increases the size of the anion increases
For instance, lithium and sodium lose the single electron from their outermost energy level to form cations. The ions so formed are smaller because the remaining electrons are at a inner cells and attracted more strongly by the nucleus. Fluorine and chlorine become negative ions by gaining an electron. When electrons are added, the charge on the nucleus is not great enough to hold the increased number of electrons as closely as it holds the electrons in the neutral atom. So, as seen in atomic radius, ionic radii also decrease along the period from left to right and increase down the group.
Ionisation Energy:
It is defined as the minimum amount of energy required to remove the most loosely bound electron from the valence shell of the isolated neutral gaseous atom in its ground state. It is expressed in kJ mol-1 or in electron volts (eV).
![]()
Where IE1 represents the first ionisation energy.
Successive Ionisation energies
The minimum amount of energy required to remove an electron from a unipositive cation is called second ionisation energy. It is represented by the following equation.
![]()
In this way we can define the successive ionisation energies such as third, fourth etc.
The total number of electrons are less in the cation than the neutral atom while the nuclear charge remains the same. Therefore the effective nuclear charge of the cation is higher than the corresponding neutral atom. Thus the successive ionisation energies, always increase in the following order
IE1 < IE2 < IE3 <…..
Ionisation energy is the minimum energy required to remove an electron from an isolated gaseous atom in its ground state to form a cation. It is otherwise called ionisation enthalpy. It is measured in kJ/mol. Higher the ionisation energy, it is more difficult to remove the electron.
As the atomic size decreases from left to right in a period, more energy is required to remove the electrons. So, the ionisation energy increases along the period. But, down the group, the atomic size increases and hence the valence electrons are loosely bound. They require relatively less energy for the removal. Thus, ionisation energy decreases down the group in the periodic table.
Periodic Trends in Ionisation Energy
The ionisation energy usually increases along a period with few exceptions. As discussed earlier, when we move from left to right along a period, the valence electrons are added to the same shell, at the same time protons are added to the nucleus. This successive increase of nuclear charge increases the electrostatic attractive force on the valence electron and more energy is required to remove the valence electron resulting in high ionisation energy.
Let us consider the variation in ionisation energy of second period elements. The plot of atomic number vs ionisation energy is given below.
In the following graph, there are two deviation in the trends of ionisiation energy. It is expected that boron has higher ionisation energy than beryllium since it has higher nuclear charge. However, the actual ionisation energies of beryllium and boron are 899 and 800 kJ mol-1 respectively contrary to the expectation. It is due to the fact that beryllium with completely filled 2s orbital, is more stable than partially filled valence shell electronic configuration of boron. (2s2, 2p1)
Variation of Ionisation energy along the II period
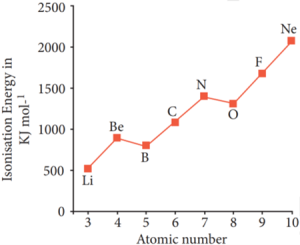
The electronic configuration of beryllium (Z=4) in its ground state is 1s2, 2s2 and that of boran is (Z = 5) 1s2 2s2 2p1
Similarly, nitrogen with 1s2, 2s2, 2p3 electronic configuration has higher ionisation energy (1402 kJ mol-1) than oxygen (1314 kJ mol-1). Since the half filled electronic configuration is more stable, it requires higher energy to remove an electron from 2p orbital of nitrogen. Whereas the removal one 2p electron from oxygen leads to a stable half filled configuration. This makes comparatively easier to remove 2p electron from oxygen.
Periodic variation in group
The ionisation energy decreases down a group. As we move down a group, the valence electron occupies new shells, the distance between the nucleus and the valence electron increases. So, the nuclear forces of attraction on valence electron decreases and hence ionisation energy also decreases down a group.
Ionisation energy and shielding effect
As we move down a group, the number of inner shell electron increases which in turn increases the repulsive force exerted by them on the valence electrons, i.e. the increased shielding effect caused by the inner electrons decreases the attractive force acting on the valence electron by the nucleus. Therefore the ionisation energy decreases.
Let us understand this trend by considering the ionisation energy of alkali metals.
Variation of Ionisation energy down the I Group.
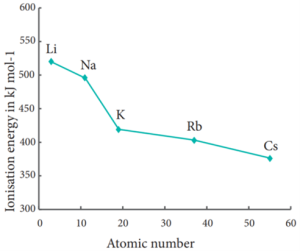
Electron Affinity:
It is defined as the amount of energy released (required in the case noble gases) when an electron is added to the valence shell of an isolated neutral gaseous atom in its ground state to form its anion. It is expressed in kJ mol-1
![]()
Variation of Electron Affinity in a period: The variation of electron affinity is not as systematic as in the case of ionisation energy. As we move from alkali metals to halogens in a period, generally electron affinity increases, i.e. the amount of energy released will be more. This is due to an increase in the nuclear charge and decrease in size of the atoms. However, in case of elements such as beryllium (1s2, 2s2), nitrogen (1s2, 2s2, 2p3) the addition of extra electron will disturb their stable electronic configuration and they have almost zero electron affinity.
Variation of electron affinity (electron gain energy) along II period
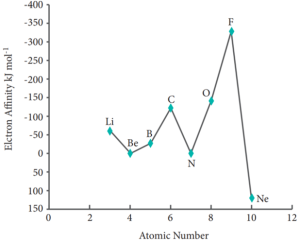
Noble gases have stable ns2, np6 configuration, and the addition of further electron is unfavourable and requires energy. Halogens having the general electronic configuration of ns2, np5 readily accept an electron to get the stable noble gas electronic configuration (ns2, np6), and therefore in each period the halogen has high electron affinity. (High negative values)
Variation of Electron affinity in a group:
As we move down a group, generally the electron affinity decreases. It is due to increase in atomic size and the shielding effect of inner shell electrons. However, oxygen and fluorine have lower affinity than sulphur and chlorine respectively. The sizes of oxygen and fluorine atoms are comparatively small and they have high electron density. Moreover, the extra electron added to oxygen and fluorine has to be accommodated in the 2p orbital which is relatively compact compared to the 3p orbital of sulphur and chlorine so, oxygen and fluorine have lower electron affinity than their respective group elements sulphur and chlorine.
Variation of Electron Affinity (electron gain energy) along 17th group
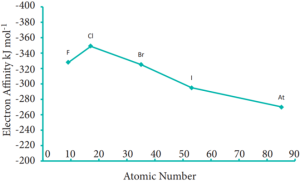
Electro negativity:
It is defined as the relative tendency of an element present in a covalently bonded molecule, to attract the shared pair of electrons towards itself.
Electro negativity is not a measurable quantity. However, a number of scales are available to calculate its value. One such method was developed by Pauling, he assigned arbitrary value of electro negativities for hydrogen and fluorine as 2.1 and 4.0 respectively. Based on this the electro negativity values for other elements can be calculated using the following expression
Where EAB, EAA and EBB are the bond dissociation energies (K cal) of AB, A2 and B2 molecules respectively.
The electro negativity of any given element is not a constant and its value depends on the element to which it is covalently bound. The electronegativity values play an important role in predicting the nature of the bond.
Variation of Electro negativity in a period:
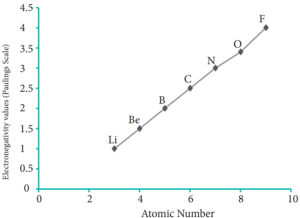
The electro negativity generally increases across a period from left to right. As discussed earlier, the atomic radius decreases in a period, as the attraction between the valence electron and the nucleus increases. Hence the tendency to attract shared pair of electrons increases. Therefore, electro negativity also increases in a period.
Variation of Electro negativity along II period
Variation of Electro negativity in a group:
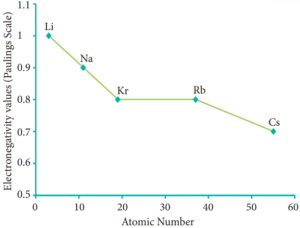
The electro negativity generally decreases down a group. As we move down a group the atomic radius increases and the nuclear attractive force on the valence electron decreases.
Hence, the electro negativity decreases. Noble gases are assigned zero electro negativity. The electro negativity values of the elements of s-block show the expected decreasing order in a group. Except 13th and 14th group all other p-block elements follow the expected decreasing trend in electro negativity.
Variation of electro negativity along I group
Variation in Periods:
Atomic radius tends to decrease in a period. As we move from left to right along a period, the valence electrons are added to the same shell. The simultaneous addition of protons to the nucleus, increases the nuclear charge, as well as the electrostatic attractive force between the valence electrons and the nucleus. Therefore atomic radius decreases along a period.
Effective nuclear charge:
In addition to the electrostatic forces of attraction between the nucleus and the electrons, there exists repulsive forces among the electrons. The repulsive force between the inner shell electrons and the valence electrons leads to a decrease in the electrostatic attractive forces acting on the valence electrons by the nucleus. Thus, the inner shell electrons act as a shield between the nucleus and the valence electrons. This effect is called shielding effect.
The net nuclear charge experienced by valence electrons in the outermost shell is called the effective nuclear charge. It is approximated by the below mentioned equation.
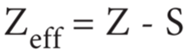
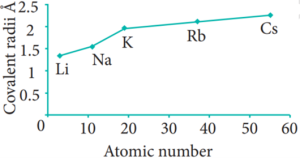
Variation in Group:
In the periodic table, the atomic radius of elements increases down the group. As we move down a group, new shells are opened to accommodate the newly added valence electrons. As a result, the distance between the centre of the nucleus and the outermost shell containing the valence electron increases. Hence, the atomic radius increases. The trend in the variation of the atomic radius of the alkali metals down the group os shown below.
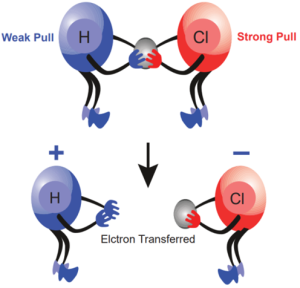
Electro negativity of an element is the measure of the tendency of its atom to attract the shared pair of electrons towards itself in a covalent bond. Let us consider HCl molecule. Both the hydrogen and chlorine atoms share one electron each to form the covalent bond between them. chlorine atom has a higher electro negativity and hence it pulls the shared electrons towards itself more strongly than hydrogen. Thus, when the bond breaks, the bonding electrons are left with chlorine forming H+ and Cl– ions. It is represented, diagrammatically, as shown below:
Electro negativity is based on various experimental data such as bond energy, ionization potential, electron affinity, etc.
Pauling scale is the widely used scale to determine the electronegativity, which in turn predicts the nature of bonding (ionic or covalent) between the atoms in a molecule.
Electro negativity of some of the elements are given below
F = 4.0, Cl = 3.0, Br = 2.8, I = 2.5, H = 2.1, Na = 1
If the difference in electro negativity between two elements is 1.7, the bond has 50% ionic character and 50% covalent character.
If the difference is less than 1.7, the bond is considered to be more covalent. If the difference is greater than 1.7, the bond is considered to be more ionic.
Along the period, from left to right in the periodic table, the electro negativity increases because of the increase in the nuclear charge which in turn attracts the electrons more strongly. On moving down a group, the electro negativity of the elements decreases because of the increased number of valence shells.
Periodic Property | In Periods | In Groups |
Atomic radius | Decreases | Increases |
Ionic radius | Decreases | Increases |
Ionisation energy | Increases | Decreases |
Electron affinity | Increases | Decreases |
Electro negativity | Increases | Decreases |
Types of Separation or Concentration of an Ore:
There are four major types of separation of ores based on the nature of the ore.
Concentration of the crushed ore is done mainly by the following methods: –
Hydraulic (Gravity Separation) method:
Principle: The difference in the densities or specific gravities of the ore and the gangue is the main principle behind this method. Oxide ores are purified by this method. e.g., Haematite Fe2 O3 the ore of iron.
Note: When the ore is heavier than the impurity, this method can be used.
Method: The ore is poured over a sloping, vibrating corrugated table with grooves and a jet of water is allowed to flow over it. The denser ore particles settle down in the grooves and lighter gangue particles are washed down by water.
Magnetic separation method:
Principle: The magnetic properties of the ores form the basis of separation. When either the ore or the gangue is magnetic, this method is employed. e.g., Tinstone SnO2 , the ore of tin.
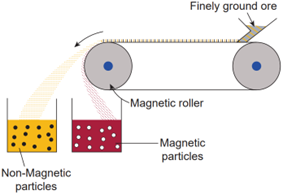
Method: The crushed ore is placed over a conveyer belt which rotates around two metal wheels, one of which is magnetic. The magnetic particles are attracted to the magnetic wheel and fall separately apart from the non-magnetic particles.
Froth floatation:
Principle: This process depends on the preferential wettability of the ore with oil (pine oil) and the gangue particles by water. Lighter ores, such as sulphide ores, are concentrated by this method. e.g., Zinc blende (ZnS).
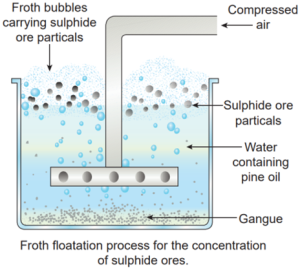
Note: When the impurity is heavier than the ore, this method can be used.
Method: The crushed ore is taken in a large tank containing pine oil and water and agitated with a current of compressed air.The ore is wetted by the oil and gets separated from the gangue in the form of froth. Since the ore is lighter, it comes on the surface with the froth and the impurities are left behind. e.g., Zinc blende (ZnS).
Chemical method or Leaching:
This method is employed when the ore is in a very pure form.
The ore is treated with a suitable reagent such that the ore is soluble in it but the impurities are not. The impurities are removed by filtration. The solution of the ore, ie., the filtrate is treated with a suitable reagent which precipitates the ore. E.g. Bauxite Al2O3 .2H2 O, (the ore of aluminium).
Types and Uses Of Iron:
Pig iron (Iron with 2.0% – 4.5% of carbon): It is used in making pipes, stoves, radiators, railings, manhole covers and drain pipes.
Steel (Iron with 0.25% – 2.0% of carbon): It is used in the construction of buildings, machinery, transmission cables and T.V towers and in making alloys.
Wrought iron (Iron with < 0.25% of carbon): It is used in making springs, anchors and electromagnets.
Alloys:
An alloy is a homogeneous mixture of two or more metals or of one or more metals with certain non-metallic elements.
The properties of alloys are often different from those of its components. Pure gold is brittle to be used. The addition of small percentage of copper enhances its strength and utility.
Amalgam:
An amalgam is an alloy of mercury with another metal. These alloys are formed through metallic bonding with the electrostatic force of attraction between the electrons and the positively charged metal ions. Silver tin amalgam is used for dental filling.
Reasons for alloying:
To modify appearance and colour
To modify chemical activity.
To lower the melting point.
To increase hardness and tensile strength.
To increase resistance to electricity.
Method of making alloys:
By fusing the metals together. E.g. Brass is made by melting zinc and copper.
By compressing finely divided metals. E.g. Wood metal: an alloy of lead, tin, bismuth and cadmium powder is a fusible alloy.
Alloys as solid solutions:
Alloys can be considered as solid solutions in which the metal with high concentration is solvent and other metals are solute. For example, brass is a solid solution of zinc (solute) in copper (solvent).
Types of Alloys:
Based on the presence of Iron, alloys can be classified into:
Ferrous alloys: Contain Iron as a major component. A few examples of ferrous alloys are Stainless Steel, Nickel Steel etc.
Non-ferrous alloys: These alloys do not contain Iron as a major component. For example, Aluminium alloy, Copper alloy etc.
Copper Alloys (Non- ferrous):
Alloys | Uses |
Brass (Cu, Zn) | Electrical fittings, medal, decorative items, hardware |
Bronze (Cu, Sn) | Statues, coins, bells, gongs |
Aluminium Alloys (Non- ferrous):
Alloys | Uses |
Duralumin (Al, Mg, Mn, Cu) | Aircrafts, tools, pressure cookers |
Magnalium (Al, Mg) | Aircraft, scientific instruments |
Iron Alloys (Ferrous):
Alloys | Uses |
Stainless steel (Fe,C, Ni,Cr) | Utensils, cutlery, automobile parts |
Nickel steel (Fe,C,Ni) | Cables , aircraftparts, propeller |
Corrosion:
It is the gradual destruction of metals by chemical or electrochemical reaction with the environment. It is a natural process which converts a metal into its oxide, hydroxide or sulphide so that it loses its metallic characteristics.
Rusting:
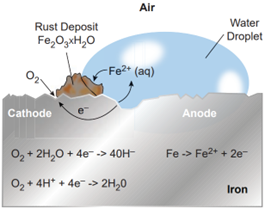
Rust is chemically known as hydrated ferric oxide (it is formulated as Fe2 O3. xH2 O). Rusting results in the formation of scaling reddish brown hydrated ferric oxide on the surface of iron and iron containing materials.
Types of Corrosion:
Dry Corrosion or Chemical Corrosion: The corrosive action in the absence of moisture is called dry corrosion. It is the process of a chemical attack on a metal by a corrosive liquids or gases such as O2, N2, SO2, H2 S etc. It occurs at high temperature. Of all the gases mentioned above O2 is the most reactive gas to impart the chemical attack.
Wet Corrosion or Electrochemical Corrosion: The corrosive action in the presence of moisture is called wet corrosion. It occurs as a result of electrochemical reaction of metal with water or aqueous solution of salt or acids or bases.
Methods of preventing corrosion:
Alloying: The metals can be alloyed to prevent from the process of corrosion. E.g: Stainless Steel
Surface Coating: It involves application of a protective coating over the metal. It is of the following types:
Galvanization: It is the process of coating zinc on iron sheets by using electric current.
Electroplating: It is a method of coating one metal over another metal by passing electric current.
Anodizing: It is an electrochemical process that converts the metal surface into a decorative, durable and corrosion resistant. Aluminium is widely used for anodizing process.
Cathodic Protection: It is the method of controlling corrosion of a metal surface protected is coated with the metal which is easily corrodible. The easily corrodible metal is called sacrificial metal to act as anode ensuring cathodic protection.
Applications of Metals:
Applications of Al:
Aluminium is the most abundant metal and is a good conductor of electricity and heat. It also resists corrosion. The following are some of its applications.
Many heat exchangers/sinks and our day to day cooking vessels are made of aluminium.
It is used as wraps (aluminium foils) and is used in packing materials for food items,
Aluminium is not very strong, However, its alloys with copper, manganese, magnesium and silicon are light weight and strong and they are used in design of aeroplanes and other forms of transport.
As Aluminium shows high resistance to corrosion, it is used in the design of chemical reactors, medical equipments, refrigeration units and gas pipelines.
Aluminium is a good electrical conductor and cheap, hence used in electrical overhead electric cables with steel core for strength.
Applications of Zn:
Metallic zinc is used in galvanising metals such as iron and steel structures to protect them from rusting and corrosion.
Zinc is also used to produce die-castings in the automobile, electrical and hardware industries.
Zinc oxide is used in the manufacture of many products such as paints, rubber, cosmetics, pharmaceuticals, plastics, inks, batteries, textiles and electrical equipment. Zinc sulphide is used in making luminous paints, fluorescent lights and x-ray screens.
Brass an alloy of zinc is used in water valves and communication equipment as it is highly resistant to corrosion.
Applications of Fe:
Iron is one of the most useful metals and its alloys are used everywhere including bridges, electricity pylons, bicycle chains, cutting tools and rifle barrels.
Cast iron is used to make pipes, valves and pumps stoves etc…
Magnets can be made from iron and its alloys and compounds.
An important alloy of iron is stainless steel, and it is very resistant to corrosion. It is used in architecture, bearings, cutlery, surgical instruments and jewellery. Nickel steel is used for making cables, automobiles and aeroplane parts. Chrome steels are used for maufacturing cutting tools and crushing machines
Applications of Cu:
Copper is the first metal used by the human and extended use of its alloy bronze resulted in a new era,’Bronze age’
Copper is used for making coins and ornaments along with gold and other metals.
Copper and its alloys are used for making wires, water pipes and other electrical parts
Applications of Au:
Gold, one of the expensive and precious metals. It is used for coinage, and has been used as standard for monetary systems in some countries.
It is used extensively in jewellery in its alloy form with copper. It is also used in electroplating to cover other metals with a thin layer of gold which are used in watches, artificial limb joints, cheap jewellery, dental fillings and electrical connectors.
Gold nanoparticles are also used for increasing the efficiency of solar cells and also used a catalysts.
