OXIDATION AND REDUCTION & CARBON AND NITROGEN AND THEIR COMPOUNDS
Oxidation, Reduction and Redox Reactions:
When an apple is cut and left for sometimes, its surface turns brown. Similarly, iron bolts and nuts in metallic structures get rusted. Do you know why these are happening? It is because of a reaction called oxidation.
Oxidation: The chemical reaction which involves addition of oxygen or removal of hydrogen or loss of electrons is called oxidation.

Reduction:
The chemical reaction which involves addition of hydrogen or removal of oxygen or gain of electrons is called reduction.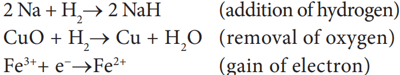
Redox reactions: Generally, the oxidation and reduction occurs in the same reaction (simultaneously). If one reactant gets oxidised, the other gets reduced. Such reactions are called oxidation-reduction reactions or Redox reactions.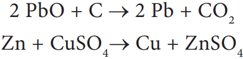
Oxidation | Addition of oxygen |
Removal of hydrogen | |
Loss of electron | |
Reduction | Removal of oxygen |
Addition of hydrogen | |
Gain of electron |
Oxidising Agents and Reducing Agents:
Substances which have the ability to oxidise other substances are called oxidising agents. These are also called as electron acceptors because they remove electrons from other substances.
Example: H2O2, MnO4 −, CrO3, Cr2 O72−
Substances which have the ability to reduce other substances are called Reducing agents. These are also called as electron donors because they donate electrons to other substances.
Example: NaBH4, LiAlH4 and metals like Palladium, Platinum.
Oxidation Reactions in Daily Life:
In nature, the oxygen present in atmospheric air oxidises many things, starting from metals to living tissues.
- The shining surface of metals tarnishes due to the formation of respective metal oxides on their surfaces. This is called corrosion.
- The freshly cut surfaces of vegetables and fruits turn brown after some time because of the oxidation of compounds present in them.
- The oxidation reaction in food materials that were left open for a long period is responsible for spoiling of food. This is called Rancidity.
Oxidation Number:
Oxidation number of an element is defined as the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. Oxidation number is also called oxidation state. If the oxidation number is positive then it means that the atom loses electron, and if it is negative it means that the atom gains electrons. If it is zero then the atom neither gains nor loses electrons. The sum of oxidation numbers of all the atoms in the formula for a neutral compound is ZERO. The sum of oxidation numbers of an ion is the same as the charge on that ion. Negative oxidation number in a compound of two unlike atoms is assigned to the more electronegative atom.
Example:
- Oxidation number of K and Br in KBr molecule is +1 and –1 respectively.
- Oxidation number of N in NH3 molecule is –3.
- Oxidation number of H is +1 (except hydrides).
- Oxidation number of oxygen in most cases is –2.
Haemoglobin and Oxygen Transport
- Even a small amount of oxygen present in air leads to the rusting of iron, i.e. iron is oxidised to Fe3+. But the Fe2+ present in haemoglobin which binds oxygen during transport of oxygen from lungs to tissues never gets oxidised. Do you know why?
- The answer lies in the structural features of haemoglobin. Haemoglobin contains four sub units each with a porphyrin ring (heme) attached to the protein (globin) molecule. In this structure, the iron (Fe2+) forms a co-ordination complex with an octahedral geometry. The four positions of the octahedron are occupied by porphyrin rings, fifth position is filled by imidazole ring of a histidine residue and the sixth position is utilized for binding the oxygen molecule. Generally the Fe2+ in heme is susceptible to oxidation. Since the Fe2+ ion in haemoglobin is surrounded by the globin protein chain that provides a hydrophobic environment, the oxidation of Fe2+ becomes difficult.
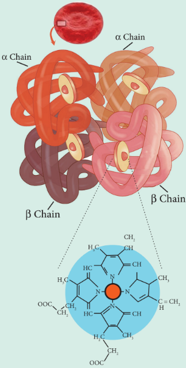
However, 3% of haemoglobin is oxidised to methemoglobin (haemoglobin where the iron is present in Fe3+ state and oxygen does not bind to this) daily. The enzyme methemoglobin reductase reduces it back to haemoglobin.
Cyanide poisoning: While oxygen binds reversibly to haemoglobin, cyanide binds irreversibly to haemoglobin and blocks oxygen binding. As a result the transport of oxygen from the lungs to tissues is stopped. It leads to the quick death of the person.
Types of Redox Reactions:
Redox reactions are classified into the following types.
- Combination reactions:
Redox reactions in which two substances combine to form a single compound are called combination reaction.
Example: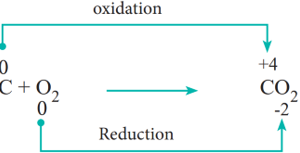
- Decomposition reactions:
Redox reactions in which a compound breaks down into two or more components are called decomposition reactions. These reactions are opposite to combination reactions. In these reactions, the oxidation number of the different elements in the same substance is changed.
Example: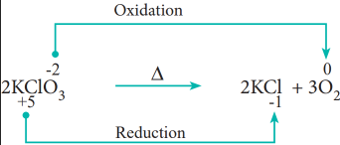
- Displacement reactions:
Redox reactions in which an ion (or an atom) in a compound is replaced by an ion (or atom) of another element are called displacement reactions. They are further classified into (i) metal displacement reactions (ii) non-metal displacement reactions.
(i) Metal displacement reactions:
Place a zinc metal strip in an aqueous copper sulphate solution taken in a beaker. Observe the solution, the intensity of blue colour of the solution slowly reduced and finally disappeared.
The zinc metal strip became coated with brownish metallic copper. This is due to the following metal displacement reaction.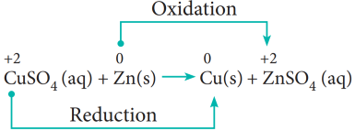
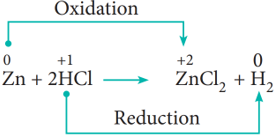
- ii) Non-metal displacement
- Disproportionation reaction (Auto redox reactions)
In some redox reactions, the same compound can undergo both oxidation and reduction. In such reactions, the oxidation state of one and the same element is both increased and decreased. These reactions are called disproportionation reactions.
Examples: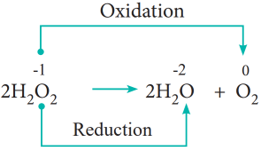
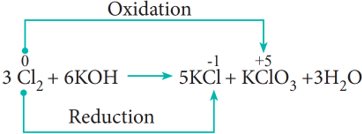
- Competitive electron transfer reaction
In metal displacement reactions, we learnt that zinc replaces copper from copper sulphate solution. Let us examine whether the reverse reaction takes place or not. As discussed earlier, place a metallic copper strip in zinc sulphate solution. If copper replaces zinc from zinc sulphate solution, Cu2+ ions would be released into the solution and the colour of the solution would change to blue. But no such change is observed. Therefore, we conclude that among zinc and copper, zinc has more tendency to release electrons and copper to accept the electrons.
Let us extend the reaction to copper metal and silver nitrate solution. Place a strip of metallic copper in sliver nitrate solution taken in a beaker. After some time, the solution slowly turns blue. This is due to the formation of Cu2+ ions, i.e. copper replaces silver from silver nitrate. The reaction is,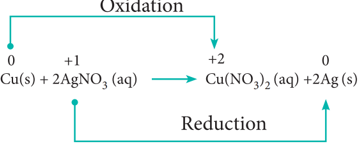
It indicates that between copper and silver, copper has the tendency to release electrons and silver to accept electrons.
From the above experimental observations, we can conclude that among the three metals, namely, zinc, copper and silver, the electron releasing tendency is in the following order.
Zinc > Copper > Silver
This kind of competition for electrons among various metals helps us to design (galvanic) cells. In XII standard we will study the galvanic cell in detail.
- Gastric Acid and Antacids
Antacids are commonly used medicines for treating heartburn and acidity. Do you know the chemistry behind it?
Gastric acid is a digestive fluid formed in the stomach and it contains hydrochloric acid. The typical concentration of the acid in gastric acid is 0.082 M. When the concentration exceeds 0.1 M it causes the heartburn and acidity.
Antacids used to treat acidity contain mostly magnesium hydroxide or aluminium hydroxide that neutralises the excess acid. The chemical reactions are as follows.![]()
![]()
From the above reactions we know that 1 mole of aluminium hydroxide neutralises 3 moles of HCl while 1 mole of magnesium hydroxide neutralises 2 moles of HCl.
Let us calculate the amount of acid neutralised by an antacid that contains 250 mg of aluminium hydroxide and 250 mg of magnesium hydroxide.
Active Compound | Mass in (mg) | Molecular mass (g mol-1) | No. of moles of active compound | No. of moles OH– |
Al(OH)3 | 250 | 78 | 0.0032 | 0.0096 |
Mg(OH)2 | 250 | 58 | 0.0043 | 0.0086 |
Total no. of moles of OH– ion from one tablet | 0.0182 | |||
One tablet of above composition will neutralise 0.0182 mole of HCl for a person with gastric acid content of 0.1 mole. One tablet can be used to neutralize the excess acid which will bring the concentration back to normal level. (0.1 – 0.018 = 0.082 M)
Carbon and Nitrogen:
1.Inorganic Carbon Compounds
Compounds | Formation | Properties | Uses |
Carbon monoxide (CO) | Not a natural component of air. Mainly added to atmosphere due to incomplete combustion of fuels. | Colourless, odourless, highly toxic, sparingly soluble in water. | Main component of water gas (CO+H2). Reducing agent. |
Carbon dioxide (CO2) | Occurs in nature as free and combined forms. Combined form is found in minerals like limestone, magnesite. Formed by complete combustion of carbon or coke | Colourless, odourless, tasteless Stable, highly soluble in water, takes part in photosynthesis. | Fire extinguisher, preservative for fruits, making bread, to manufacture urea, carbonated water, nitrogenous fertilizers, dry ice in refrigerator |
Calcium Carbide (CaC2) | Prepared by heating calcium oxide and coke. | Greyish black solid. | To manufacture graphite and hydrogen. To prepare acetylene gas for welding. |
Carbon disulphide (CS2) | Directly prepared from Carbon and Sulphur | Colourless, inflammable, highly poisonous gas. | Solvent for sulphur. To manufacture rayon, fungicide, insecticide |
Calcium Carbonate (CaCO3 ) | Prepared by passing Carbondioxide into the solution of slaked lime | Crystalline solid, insoluble in water. | Antacid |
Sodium bicarbonate (NaHCO3 ) | Formed by treating sodium hidroxide with carbonic acid (H2 CO3) | White crystalline substance, sparingly soluble in water | Preparation of sodium carbonate, baking powder, antacid |
- Carbon compounds in everyday life
- It is impossible to think of our daily life without carbon compounds. Over time, a large number of carbon compounds have been developed for the improvement of our lifestyle and comfort. They include carbonbased fuels, carbon nanomaterials, plastics, carbon filters, carbon steel, etc.
- Even though carbon and its compounds are vital for modern life, some of its compounds like CO, cyanide and certain types of plastics are harmful to humans. In the following segment, let us discuss the role of plastics in our daily life and how we can become aware of the toxic chemicals that some plastics contain.
- Plastics – Catenated long chain carbon compounds
- Plastics are a major class of catenated organic carbon compounds. They are made from long chain organic compounds called ‘polymer resins’ with chemical additives that give them different properties. Different kinds of polymers are used to make different types of plastics. Plastics are everywhere. They are convenient, cheap and are used in our everyday life. Plastics have changed the way we live. They have helped improve health care, transport and food safety. Plastics have allowed many breakthroughs in technologies such as smartphones, computers and the internet. It is clear that plastic has given our society many benefits. But these benefits have come at a cost.
Drawbacks of plastics
- Plastics take a very long time to fully break down in nature.
- The microbes that break down plastic are too few in nature to deal with the quantity of plastics we produce.
- A lot of plastic does not get recycled and ends up polluting the environment.
- Some types of plastics contain harmful chemical additives that are not good for human health.
- Burning of plastics releases toxic gases that are harmful to our health and contribute to climate change.
- One-time use and throwaway plastics end up littering and polluting the environment.
In order to know which plastics are harmful, you will need to learn the secret ‘language’ of plastics (resin codes).
Identifying different types of plastics:
(a) The resin codes
- Look at the following pictures. One is a plastic sachet in which milk is distributed to consumers and the other is a plastic food container. Observe the code shown on it (circled). Do you know what this code means? It is called a ‘resin code’. The resin code represents the type of polymer used to make the plastic.
(b) Need for resin codes
- Plastics should be recycled or disposed of safely. Certain types of plastics should be avoided so that they do not end up polluting the environment or harming our health. Each plastic is composed of a different polymer or set of molecules. Different molecules do not mix when plastics are recycled, it is like trying to recycle paper and glass together. For this reason, they need to be separated. The resin codes of plastics were designed in 1988 and are a uniform way of classifying the different types of plastic which help recyclers in the sorting process.
(c) Find in the resin code on plastic items
- The secret resin codes are shown as three chasing arrows in a triangle. There is a number in the middle or letters under the triangle (an acronym of that plastic type). This is usually difficult to find. It can be found on the label or bottom of a plastic item.
- The resin codes are numbered from 1 to 7. Resin codes #1 to #6 each identify a certain type of plastic that is often used in products. Resin code #7 is a category which is used for every other plastic (since 1988) that does not fit into the categories #1 to #6. The resin codes look very similar to the recycling symbol, but this does not mean that all plastics with a code can be recycled.
(d) Where will the resin code be shown on plastic items?
- Flip a plastic item to find the resin code on the bottom.
- Sometimes the bottom of plastic item will only have an acronym or the full name of that plastic type.
- If you do not find it on the bottom, search for the code on the label.
- Some plastics do not have a code. The company did not follow the rules and you do not know if it is safe to use.
Harmful effects of plastics
- Plastics in our everyday life can be harmful for two reasons. The first reason is that some types of plastic contain chemicals that are harmful to our health. The second reason is that a lot of plastics are designed to be used just for one time. This use and throwaway plastic cause’s pollution to our environment.
(a) Harmful plastics
- There are three types of plastic that use toxic and harmful chemicals. These chemicals are added to plastics to give them certain qualities such as flexibility, strength, colour and fire or UV resistance. The three unsafe plastics are: PVC (resin code #3), PS (resin code #6 also commonly called Thermocol) and PC/ABS (resin code #7).
PVC – Polyvinyl Chloride plastics
- Heavy metals (cadmium & lead) are added to PVC.
- Phthalates (chemical additive) copy our hormones.
- Burning PVC releases dioxins (one of the most toxic chemicals known to humans).
PS – Polystyrene plastics
- Styrene is a building block of this plastic and may cause cancer.
- It takes very long time to break-down (100- 1 million years).
- Higher amounts of toxic styrene leak into our food and drinks when they are hot or oily.
PC – Polycarbonate plastics
- PC plastic contains Bisphenol A (BPA).
- BPA leaks out of PC products used for food and drinks.
- BPA increases or decreases certain hormones and changes the way our bodies work.
ABS – Acrylonitrile Butadiene Styrene
- Styrene causes problems for our eyes, skin, digestive system and lungs.
- Brominated Flame Retardants (BFRs) are often added.
- Studies show that toxic chemicals leak from this plastic.
(b) One-time use plastic
- Use and throwaway plastics cause short and long-term environmental damage. Half of all the plastic made today is used for throwaway plastic items. These block drains and pollute water bodies. One-time use plastic causes health problems for humans, plants and animals. Some examples are plastic carry bags, cups, plates, straws, water pouches, cutlery and plastic sheets used for food wrapping.
- These items take a few seconds to be made in a factory. You will use them for a very short time. Once you throw them away, they can stay in our environment for over a 1,000 years causing plastic pollution for future generations. We need rules and laws to protect people and the environment from plastic pollution.
Homologous Series:
- Homologous series is a group or a class of organic compounds having same general formula and similar chemical properties in which the successive members differ by a – CH2
Their condensed structural formulas are given below:
Methane – CH4
Ethane – CH3CH3
Propane – CH3CH2CH3
Butane – CH3 (CH2)2CH3
Pentane – CH3 (CH2) 3CH3
If you observe the above series. You can notice that each successive member has one methylene group more than the precedent member of the series and hence they are called homologs.
Characteristics of homologous series
- Each member of the series differs from the preceding or succeeding member by one methylene group (–CH2) and hence by a molecular mass of 14 amu.
- All members of a homologous series contain the same elements and functional group.
- They are represented by a general molecular formula. e.g. Alkanes, CnH2n + 2.
- The members in each homologous series show a regular gradation in their physical properties with respect to their increase in molecular mass.
- Chemical properties of the members of a homologous series are similar.
- All the members can be prepared by a common method.
Ethanol (CH3CH2OH):
Ethanol is commonly known as alcohol. All alcoholic beverages and some cough syrups contain ethanol. Its molecular formula is C2H5OH. Its structural formula is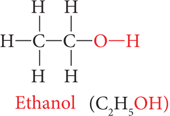
Manufacture of ethanol:
- Ethanol is manufactured in industries by the fermentation of molasses, which is a by-product obtained during the manufacture of sugar from sugarcane. Molasses is a dark coloured syrupy liquid left after the crystallization of sugar from the concentrated sugarcane juice. Molasses contain about 30% of sucrose, which cannot be separated by crystallization. It is converted into ethanol by the following steps:
(i) Dilution of molasses
- Molasses is first diluted with water to bring down the concentration of sugar to about 8 to 10 percent.
(ii) Addition of Nitrogen source
- Molasses usually contains enough nitrogenous matter to act as food for yeast during the fermentation process. If the nitrogen content of the molasses is poor, it may be fortified by the addition of ammonium sulphate or ammonium phosphate.
(iii) Addition of Yeast
- The solution obtained in step (ii) is collected in large ‘fermentation tanks’ and yeast is added to it. The mixture is kept at about 303K for a few days. During this period, the enzymes invertase and zymase present in yeast, bring about the conversion of sucrose into ethanol.
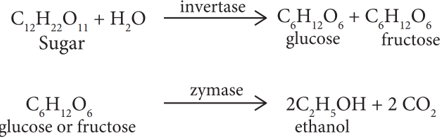
The fermented liquid is technically called wash.
(iv) Distillation of ‘Wash’
- The fermented liquid (i.e. wash), containing 15 to 18 percent alcohol, is now subjected to fractional distillation. The main fraction drawn is an aqueous solution of ethanol which contains 95.5% of ethanol and 4.5% of water. This is called rectified spirit. This mixture is then refluxed over quicklime for about 5 to 6 hours and then allowed to stand for 12 hours. On distillation of this mixture, pure alcohol (100%) is obtained. This is called absolute alcohol.
Physical properties:
- Ethanol is a colourless liquid, having a pleasant smell and a burning taste.
- It is a volatile liquid. Its boiling point is 780 C (351K), which is much higher than that of its corresponding alkane, i.e. ethane (Boiling Point = 184 K).
- It is completely miscible with water in all proportions.
Chemical Properties:
(i) Dehydration (Loss of water)
When ethanol is heated with con H2SO4 at 443K, it loses a water molecule i.e. dehydrated to form ethene.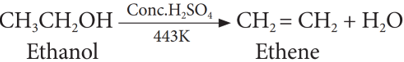
(ii) Reaction with sodium:
Ethanol reacts with sodium metal to form sodium ethoxide and hydrogen gas.![]()
(iii) Oxidation:
Ethanol is oxidized to ethanoic acid with alkaline KMnO4 or acidified K2Cr2O7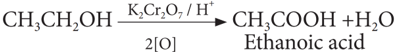
During this reaction, the orange colour of K2Cr2O7 changes to green. Therefore, this reaction can be used for the identification of alcohols.
(iv) Esterification:
The reaction of an alcohol with a carboxylic acid gives a compound having fruity odour. This compound is called an ester and the reaction is called esterification. Ethanol reacts with ethanoic acid in the presence of conc. H2SO4 to form ethyl ethanoate, an ester.
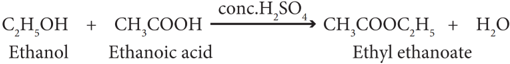
(v) Dehydrogenation:
When the vapour of ethanol is passed over heated copper, used as a catalyst at 573 K, it is dehydrogenated to acetaldehyde.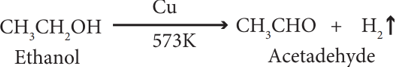
(vi) Combustion:
Ethanol is highly inflammable liquid. It burns with oxygen to form carbon dioxide and water.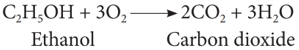
Uses of ethanol:
Ethanol is used
- In medical wipes, as an antiseptic.
- As an anti-freeze in automobile radiators.
- For effectively killing microorganisms like bacteria, fungi, etc., by including it in many hand sanitizers.
- As an antiseptic to sterilize wounds in hospitals.
- As a solvent for drugs, oils, fats, perfumes, dyes, etc.
- In the preparation of methylated spirit (mixture of 95% of ethanol and 5% of methanol) rectified spirit (mixture of 95.5% of ethanol and 4.5% of water), power alcohol (mixture of petrol and ethanol) and denatured spirit (ethanol mixed with pyridine).
- To enhance the flavour of food extracts, for example vanilla extract; a common food flavour, which is made by processing vanilla beans in a solution of ethanol and water.
Organic Compounds in Daily Life:
Organic compounds are inseparable in human life. They are used by mankind or associated at all stages of life right from one’s birth to death. Various classes of organic compounds and their uses in our daily life as follows:
Hydrocarbons
- Fuels like LPG, Petrol, and Kerosene.
- Raw materials for various important synthetic materials.
- Polymeric materials like tyre, plastic containers.
Alcohols
- As a solvent and an antiseptic agent.
- Raw materials for various important synthetic materials.
Aldehydes
- Formaldehyde as a disinfectant.
- Raw materials for synthetic materials.
Ketones
- As a solvent.
- Stain Remover.
Ethers
- Anaesthetic agents.
- Pain Killer.
Esters
- All the cooking oils and lipids contain esters.
Soaps and Detergents:
Soaps and the Detergents are materials that are used by us for cleaning purposes because pure water alone cannot remove all types of dirt or any oily substance from our body or clothes. They contain ‘surfactants’, which are compounds with molecules that line up around water to break the ‘surface tension’. Both of them having a different chemical nature. Soap is a cleaning agent that is composed of one or more salts of fatty acids. Detergent is a chemical compound or a mixture of chemical compounds, which is used as a cleaning agent, also. They perform their cleaning actions in certain specific conditions. You will learn more about this in detail, in the following units.
Soap
Soaps are sodium or potassium salts of some long chain carboxylic acids, called fatty acids. Soap requires two major raw materials: i) fat and ii) alkali. The alkali, most commonly used in the preparation of soap is sodium hydroxide. Potassium hydroxide can also be used. A potassium-based soap creates a more water-soluble product than a sodium-based soap. Based on these features, there are two types of soaps:
- Hard Soap
Soaps, which are prepared by the saponification of oils or fats with caustic soda (sodium hydroxide), are known as hard soaps. They are usually used for washing purposes.
- Soft Soap
Soaps, which are prepared by the saponification of oils or fats with potassium salts, are known as soft soaps. They are used for cleansing the body.
Manufacture of soap
Kettle Process:
This is the oldest method. But, it is still widely used in the small scale preparation of soap. There are mainly, two steps to be followed in this process.
- i) Saponification of oil:
The oil, which is used in this process, is taken in an iron tank (kettle). The alkaline solution (10%) is added into the kettle, a little in excess. The mixture is boiled by passing steam through it. The oil gets hydrolysed after several hours of boiling. This process is called Saponification.
- ii) Salting out of soap:
Common salt is then added to the boiling mixture. Soap is finally precipitated in the tank. After several hours the soap rises to the top of the liquid as a ‘curdy mass’. The neat soap is taken off from the top. It is then allowed to cool down.
Effect of hard water on soap
Hard water contains calcium and magnesium ions (Ca2+ and Mg2+) that limit the cleaning action of soap. When combined with soap, hard water develops a thin layer (precipitates of the metal ions) called ‘scum’, which leaves a deposit on the clothes or skin and does not easily rinse away. Over time, this can lead to the deterioration of the fabric and eventually ruin the clothes. On the other hand, detergents are made with chemicals that are not affected by hard water.
Detergents
Development of synthetic detergents is a big achievement in the field of cleansing.
These soaps possess the desirable properties of ordinary soaps and also can be used with hard water and in acidic solutions. These are salts of sulphonic acids or alkyl hydrogen sulphates in comparison to soap, which are salts of carboxylic acids. The detergents do not form precipitates with Ca2+ and Mg2+ present in hard water. So, the cleansing action of detergents is better than that of soaps.
Preparation of detergents
Detergents are prepared by adding sulphuric acid to the processed hydrocarbon obtained from petroleum. This chemical reaction result in the formation of molecules similar to the fatty acid in soap. Then, an alkali is added to the mixture to produce the ‘surfactant molecules’, which do not bond with the minerals present in the hard water, thus preventing the formation of their precipitates.
In addition to a ‘surfactant’, the modern detergent contains several other ingredients. They are listed as follows:
- Sodium silicate, which prevents the corrosion and ensures that the detergent does not damage the washing machine.
- Fluorescent whitening agents that give a glow to the clothes.
- Oxygen bleaches, such as ‘sodium perborate’, enable the removal of certain stains from the cloth.
- Sodium sulphate is added to prevent the caking of the detergent powder.
- Enzymes are added to break down some stains caused by biological substances like blood and vegetable juice.
- Certain chemicals that give out a pleasant smell are also added to make the clothes fragrant after they are washed with detergents.
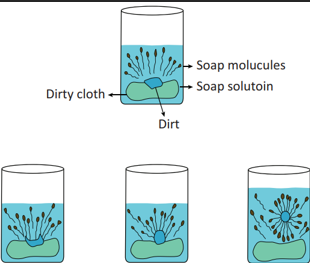
Cleansing action of soap
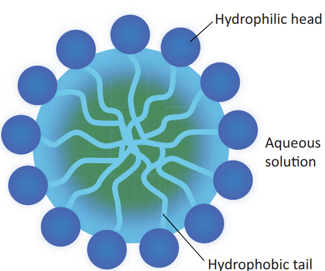
A soap molecule contains two chemically distinct parts that interact differently with water. It has one polar end, which is a short head with a carboxylate group (–COONa) and one non-polar end having the long tail made of the hydrocarbon chain.
The polar end is hydrophilic (Water loving) in nature and this end is attracted towards water. The non-polar end is hydrophobic (Water hating) in nature and it is attracted towards dirt or oil on the cloth, but not attracted towards water. Thus, the hydrophobic part of the soap molecule traps the dirt and the hydrophilic part makes the entire molecule soluble in water.
When a soap or detergent is dissolved in water, the molecules join together as clusters called ‘micelles’. Their long hydrocarbon chains attach themselves to the oil and dirt. The dirt is thus surrounded by the non-polar end of the soap molecules. The charged carboxylate end of the soap molecules makes the micelles soluble in water. Thus, the dirt is washed away with the soap.
Advantages of detergents over soaps
Detergents are better than soaps because they:
- Can be used in both hard and soft water and can clean more effectively in hard water than soap.
- Can also be used in saline and acidic water.
- Do not leave any soap scum on the tub or clothes.
- Dissolve freely even in cool water and rinse freely in hard water.
- Can be used for washing woollen garments, where as soap cannot be used.
- Have a linear hydrocarbon chain, which is biodegradable.
- Are active emulsifiers of motor grease?
- Do an effective and safe cleansing, keeping even synthetic fabrics brighter and whiter.
Biodegradable and Non-biodegradable detergents:
- a) Biodegradable detergents:
They have straight hydrocarbon chains, which can be easily degraded by bacteria.
- b) Non-biodegradable detergents:
They have highly branched hydrocarbon chains, which cannot be degraded by bacteria.
Disadvantages of Detergents
- Some detergents having a branched hydro-carbon chain are not fully biodegradable by micro-organisms present in water. So, they cause water pollution.
- They are relatively more expensive than soap.
Comparison Between Soap and Detergents:
Soap | Detergent |
It is a sodium salt of long chain fatty acids. | It is sodium salts of sulphonic acids. |
The ionic part of a soap is –COO– Na+. | The ionic part in a detergent is –SO– 3Na+. |
It is prepared from animal fats or vegetable oils. | It is prepared from hydrocarbons obtained from crude oil. |
Its effectiveness is reduced when used in hard water. | It is effective even in hard water. |
It forms a scum in hard water. | Does not form a scum in hard water. |
It has poor foaming capacity. | It has rich foaming capacity. |
Soaps are biodegradable. | Most of the detergents are non-biodegradable. |
Allotropes of carbon:
Carbon exists in many allotropic forms. Graphite and diamond are the most common allotropes. Other important allotropes are graphene, fullerenes and carbon nanotubes.
Graphite is the most stable allotropic form of carbon at normal temperature and pressure. It is soft and conducts electricity. It is composed of flat two dimensional sheets of carbon atoms. Each sheet is a hexagonal net of sp2 hybridised carbon atoms with a C-C bond length of 1.41 Å which is close to the C-C bond distance in benzene (1.40 Å). Each carbon atom forms three σ bonds with three neighbouring carbon atoms using three of its valence electrons and the fourth electron present in the unhybridised p orbital forms a π-bond. These π electrons are delocalised over the entire sheet which is responsible for its electrical conductivity. The successive carbon sheets are held together by weak vander Waals forces. The distance between successive sheet is 3.40 Å. It is used as a lubricant either on its own or as a graphited oil.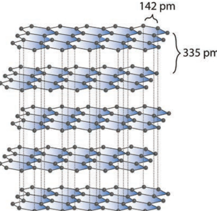
Unlike graphite the other allotrope diamond is very hard. The carbon atoms in diamond are sp3 hybridised and bonded to four neighbouring carbon atoms by σ bonds with a C-C bond length of 1.54 Å. This results in a tetrahedral arrangement around each carbon atom that extends to the entire lattice. Since all four valance electrons of carbon are involved in bonding there is no free electrons for conductivity. Being the hardest element, it used for sharpening hard tools, cutting glasses, making bores and rock drilling.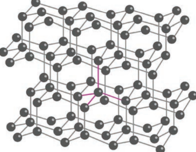
Fullerenes are newly synthesised allotropes of carbon. Unlike graphite and diamond, these allotropes are discrete molecules such as C32, C50, C60, C70, C76 etc.. These molecules have cage like structures as shown in the figure. The C60 molecules have a soccer ball like structure and is called buckminster fullerene or buckyballs. It has a fused ring structure consists of 20 six membered rings and 12 five membered rings. Each carbon atom is sp2 hybridised and forms three σ bonds & a delocalised π bond giving aromatic character to these molecules. The C-C bond distance is 1.44 Å and C=C distance 1.38 Å.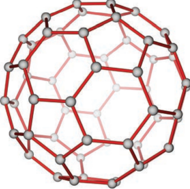
Carbon nanotubes, another recently discovered allotropes, have graphite like tubes with fullerene ends. Along the axis, these nanotubes are stronger than steel and conduct electricity. These have many applications in nanoscale electronics, catalysis, polymers and medicine.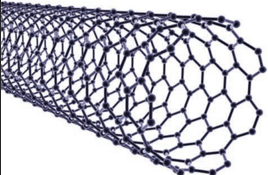
Another allotrophic form of carbon is graphene. It has a single planar sheet of sp2 hybridised carbon atoms that are densely packed in a honeycomb crystal lattice.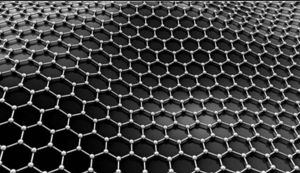
Carbon monoxide [CO]:
Preparation: Carbon monoxide can be prepared by the reaction of carbon with limited amount of oxygen.![]()
On industrial scale carbon monoxide is produced by the reaction of carbon with air. The carbon monoxide formed will contain nitrogen gas also and the mixture of nitrogen and carbon monoxide is called producer gas.![]()
The producer gas is then passed through a solution of copper (I) chloride under pressure which results in the formation of CuCl (CO).2H2 O. At reduced pressures this solution releases the pure carbon monoxide.
Pure carbon monoxide is prepared by warming methanoic acid with concentrated sulphuric acid which acts as a dehydrating agent.![]()
Properties
It is a colourless, odourless, and poisonous gas.
It is slightly soluble in water. It burns in air with a blue flame forming carbon dioxide.![]()
When carbon monoxide is treated with chlorine in presence of light or charcoal, it forms a poisonous gas carbonyl chloride, which is also known as phosgene. It is used in the synthesis of isocyanates.![]()
Carbon monoxide acts as a strong reducing agent.![]()
Under high temperature and pressure a mixture of carbon monoxide and hydrogen (synthetic gas or syn gas) gives methanol.![]()
In oxo process, ethene is mixed with carbon monoxide and hydrogen gas to produce propanal.
![]()
Fischer Tropsch synthesis:
The reaction of carbon monoxide with hydrogen at a pressure of less than 50 atm using metal catalysts at 500 – 700 K yields saturated and unsaturated hydrocarbons.![]()
Carbon monoxide forms numerous complex compounds with transition metals in which the transition meal is in zero oxidation state. These compounds are obtained by heating the metal with carbon monoxide.
Eg. Nickel tetracarbonyl [Ni(CO)4 ], Iron pentacarbonyl [Fe(CO)5 ], Chromium hexacarbonyl [Cr(CO)6 ].
Structure:
It has a linear structure. In carbon monoxide, three electron pairs are shared between carbon and oxygen. The bonding can be explained using molecular orbital theory as discussed in XI standard. The C-O bond distance is 1.128Å. The structure can be considered as the resonance hybrid of the following two canonical forms.![]()
Uses of carbon monoxide:
- Equimolar mixture of hydrogen and carbon monoxide – water gas and the mixture of carbon monoxide and nitrogen – producer gas are important industrial fuels
- Carbon monoxide is a good reducing agent and can reduce many metal oxides to metals.
- Carbon monoixde is an important ligand and forms carbonyl compound with transition metals.
Carbon dioxide:
Carbon dioxide occurs in nature in Free State as well as in the combined state. It is a constituent of air (0.03%). It occurs in rock as calcium carbonate and magnesium carbonate.
Production
On industrial scale it is produced by burning coke in excess of air.![]()
Calcination of lime produces carbon dioxide as by product.![]()
Carbon dioxide is prepared in laboratory by the action of dilute hydrochloric acid on metal carbonates.![]()
Properties
It is a colourless, nonflammable gas and is heavier than air. Its critical temperature is 31⁰ C and can be readily liquefied.
Carbon dioxide is a very stable compound. Even at 3100 K only 76 % decomposes to form carbon monoxide and oxygen. At still higher temperature it decomposes into carbon and oxygen.
Oxidising behaviour:
At elevated temperatures, it acts as a strong oxidising agent. For example,
![]()
Water gas equilibrium:
The equilibrium involved in the reaction between carbon dioxide and hydrogen, has many industrial applications and is called water gas equilibrium.
![]()
Acidic behaviour:
The aqueous solution of carbon dioxide is slightly acidic as it forms carbonic acid.
![]()
Structure of carbon dioxide
Carbon dioxide has a linear structure with equal bond distance for the both C-O bonds. In this molecule there is two C-O sigma bond. In addition there is 3c-4e bond covering all the three atoms.
![]()
Uses of carbon dioxide
- Carbon dioxide is used to produce an inert atomosphere for chemical processing.
- Biologically, it is important for photosynthesis.
- It is also used as fire extinguisher and as a propellent gas.
- It is used in the production of carbonated beverages and in the production of foam.
Boron Neutron Capture Therapy:
The affinity of Boron-10 for neutrons is the basis of a technique known as boron neutron capture therapy (BNCT) for treating patients suffering from brain tumours.
It is based on the nuclear reaction that occurs when boron-10 is irradiated with low-energy thermal neutrons to give high linear energy α particles and a Li particle.
Boron compounds are injected into a patient with a brain tumour and the compounds collect preferentially in the tumour. The tumour area is then irradiated with thermal neutrons and results in the release of an alpha particle that damages the tissue in the tumour each time a boron-10 nucleus captures a neutron. In this way damage can be limited preferentially to the tumour, leaving the normal brain tissue less affected. BNCT has also been studied as a treatment for several other tumours of the head and neck, the breast, the prostate, the bladder, andthe liver.
Nitrogen:
Preparation:
Nitrogen, the principal gas of atmosphere (78 % by volume) is separated industrially from liquid air by fractional distillation
Pure nitrogen gas can be obtained by the thermal decomposition of sodium azide about 575 K
![]()
It can also be obtained by oxidising ammonia using bromine water
![]()
Properties
Nitrogen gas is rather inert. Terrestrial nitrogen contains 14.5% and 0.4% of nitrogen-14 and nitrogen-15 respectively. The later is used for isotopic labelling. The chemically inert character of nitrogen is largely due to high bonding energy of the molecules 225 cal mol-1 (946 kJ mol-1). Interestingly the triply bonded species is notable for its less reactivity in comparison with other iso-electronic triply bonded systems such as -C≡C-, C≡O, X-C≡N, X-N≡C, -C≡C-, and -C≡N. These groups can act as donor where as dinitrogen cannot. However, it can form complexes with metal (M← N≡N) like CO to a less extent
The only reaction of nitrogen at room temperature is with lithium forming Li3N. With other elements, nitrogen combines only at elevated temperatures. Group 2 metals and Th forms ionic nitrides.
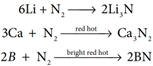
Direct reaction with hydrogen gives ammonia. This reaction is favoured by high pressures and at optimum temperature in presence of iron catalyst. This reaction is the basis of Haber’s process for the synthesis of ammonia.
![]()
With oxygen, nitrogen produces nitrous oxide at high temperatures. Even at 3473 K nitrous oxide yield is only 4.4%.
![]()
Uses of nitrogen
- Nitrogen is used for the manufacture of ammonia, nitric acid and calcium cyanamide etc.
- Liquid nitrogen is used for producing low temperature required in cryosurgery, and so in biological preservation.
Ammonia (NH3):
Preparation:
Ammonia is formed by the hydrolysis of urea.
![]()
Ammonia is prepared in the laboratory by heating an ammonium salt with a base.
![]()
It can also be prepared by heating a metal nitrides such as magnesium nitride with water.
![]()
It is industrially manufactured by passing nitrogen and hydrogen over iron catalyst (a small amount of K2 O and Al2O3 is also used to increase the rate of attainment of equilibrium) at 750 K at 200 atm pressure. In the actual process the hydrogen required is obtained from water gas and nitrogen from fractional distillation of liquid air.
Properties
Ammonia is a pungent smelling gas and is lighter than air. It can readily liquefied by at about 9 atmospheric pressure. The liquid boils at -38.4°C and freezes at -77° C. Liquid ammonia resembles water in its physical properties. i.e. it is highly associated through strong hydrogen bonding. Ammonia is extremely soluble in water (702 Volume in 1 Volume of water) at 20°C and 760mm pressure.
At low temperatures two soluble hydrate NH3 .H2 O and 2NH3 .H2 O are isolated. In these molecules ammonia and water are linked by hydrogen bonds. In aqueous solutions also ammonia may be hydrated in a similar manner and we call the same as (NH3 .H2 O)
![]()
The dielectric constant of ammonia is considerably high to make it a fairly good ionising solvent like water.
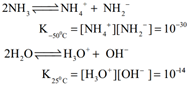
Chemical Properties
Action of heat: Above 500°C ammonia decomposes into its elements. The decomposition may be accelerated by metallic catalysts like Nickel, Iron. Almost complete dissociation occurs on continuous sparking.
![]()
Reaction with air/oxygen: Ammonia does not burn in air but burns freely in free oxygen with a yellowish flame to give nitrogen and steam.
![]()
In presence of catalyst like platinum, it burns to produce nitric oxide. This process is used for the manufacture of nitric acid and is known as Ostwald’s process.
![]()
Reducing property: Ammonia acts as a reducing agent. It reduces the metal oxides to metal when passed over heated metallic oxide.
![]()
Reaction with acids: When treated with acids it forms ammonium salts. This reaction shows that the affinity of ammonia for proton is greater than that of water.
Reaction with chlorine and chlorides: Ammonia reacts with chlorine and chlorides to give ammonium chloride as a final product. The reactions are different under different conditions as given below.
With excess ammonia
![]()
With excess of chlorine ammonia reacts to give nitrogen trichloride, an explosive substance.
![]()
Formation of amides and nitrides: With strong electro positive metals such as sodium, ammonia forms amides while it forms nitrides with metals like magnesium.
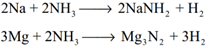
With metallic salts: Ammonia reacts with metallic salts to give metal hydroxides (in case of Fe) or forming complexes (in case Cu)
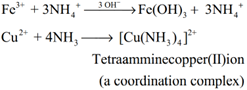
Formation of amines: Ammonia forms ammonated compounds by ion dipole attraction. Eg. [CaCl2 .8NH3]. In this, the negative ends of ammonia dipole is attracted to Ca2+ ion.
It can also act as a ligand and form coordination compounds such as [Co (NH3)6]3+, [Ag (NH3)2 ]+. For example when excess ammonia is added to aqueous solution copper sulphate a deep blue colour compound [Cu (NH3)4]2+ is formed.
Structure of ammonia Ammonia molecule is pyramidal in shape N-H bond distance is 1.016 Å and H-H bond distance is 1.645 Å with a bond angle 107°. The structure of ammonia may be regarded as a tetrahedral with one lone pair of electrons in one tetrahedral position hence it has a pyramidal shape as shown in the figure.
Nitric acid:
Preparation
Nitric acid is prepared by heating equal amounts of potassium or sodium nitrate with concentrated sulphuric acid.
![]()
The temperature is kept as low as possible to avoid decomposition of nitric acid. The acid condenses to a fuming liquid which is coloured brown by the presence of a little nitrogen dioxide which is formed due to the decomposition of nitric acid.
![]()
Commercial method of preparation
Nitric acid prepared in large scales using Ostwald’s process. In this method ammonia from Haber’s process is mixed about 10 times of air. This mixture is preheated and passed into the catalyst chamber where they come in contact with platinum gauze. The temperature rises to about 1275 K and the metallic gauze brings about the rapid catalytic oxidation of ammonia resulting in the formation of NO, which then oxidised to nitrogen dioxide.
![]()
The nitrogen dioxide produced is passed through a series of adsorption towers. It reacts with water to give nitric acid. Nitric oxide formed is bleached by blowing air.
![]()
Properties
Pure nitric acid is colourless. It boils at 86 °C. The acid is completely miscible with water forming a constant boiling mixture (98% HNO3, Boiling point 120.5 °C). Fuming nitric acid contains oxides of nitrogen. It decomposes on exposure to sunlight or on being heated, into nitrogen dioxide, water and oxygen.
![]()
Due to this reaction pure acid or its concentrated solution becomes yellow on standing. In most of the reactions, nitric acid acts as an oxidising agent. Hence the oxidation state changes from +5 to a lower one. It doesn’t yield hydrogen in its reaction with metals. Nitric acid can act as an acid, an oxidizing agent and a nitrating agent.
As an acid: Like other acids it reacts with bases and basic oxides to form salts and water
![]()
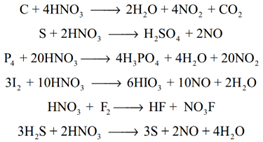
As an oxidising agent: The nonmetals like carbon, sulphur, phosphorus and iodine are oxidised by nitric acid.
As an nitrating agent: In organic compounds replacement of a –H atom with –NO2 is often referred as nitration. For example.
![]()
Nitration takes place due to the formation of nitronium ion
![]()
Action of nitric acid on metals
All metals with the exception of gold, platinum, rhodium, iridium and tantalum reacts with nitric acid. Nitric acid oxidises the metals. Some metals such as aluminium, iron, cobalt, nickel and chromium are rendered passive in concentrated acid due to the formation of a layer of their oxides on the metal surface, which prevents the nitric acid from reacting with pure metal.
With weak electropositive metals like tin, arsenic, antimony, tungsten and molybdenum, nitric acid gives metal oxides in which the metal is in the higher oxidation state and the acid is reduced to a lower oxidation state. The most common products evolved when nitric acid reacts with a metal are gases NO2, NO and H2O. Occasionally N2, NH2 OH and NH3 are also formed.
![]()
The reactions of metals with nitric acid are explained in 3 steps as follows:
Primary reaction: Metal nitrate is formed with the release of nascent hydrogen
![]()
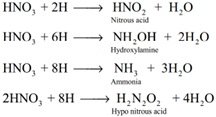
Secondary reaction: Nascent hydrogen produces the reduction products of nitric acid.
Tertiary reaction: The secondary products either decompose or react to give final products
Decomposition of the secondary:
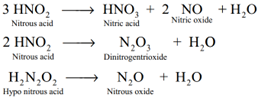
Reaction of secondary products:
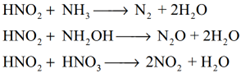
Examples:
Copper reacts with nitric acid in the following manner
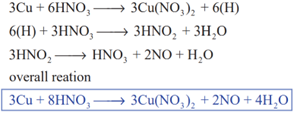
The concentrated acid has a tendency to form nitrogen dioxide
![]()
Magnesium reacts with nitric acid in the following way
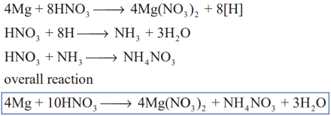
If the acid is diluted we get N2O
![]()
Uses of nitric acid:
- Nitric acid is used as a oxidising agent and in the preparation of aquaregia.
- Salts of nitric acid are used in photography (AgNO3) and gunpowder for firearms. (NaNO3)
